- 메뉴 바로가기
- 주메뉴 바로가기
- 컨텐츠 바로가기
서브컨텐츠
R&D Headquarter
You can learn more about the Huons R&D achievement and the status of new product development.
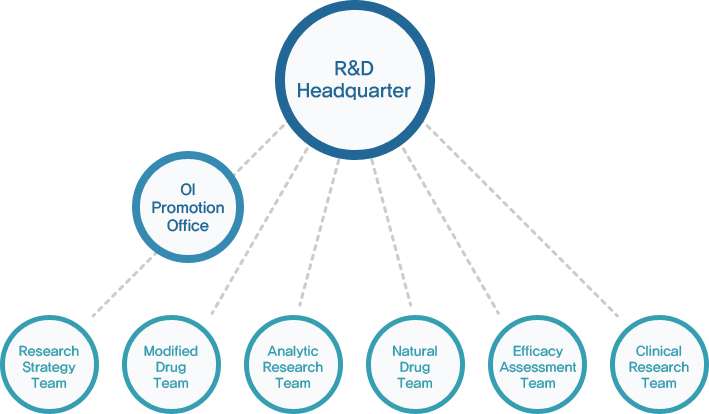
- OI Driving Team/ Research Strategy Team :
-
provides the supports for smooth research process, designs strategic plans based on previous research and
patent strategy, and explore research themes that Huons can select and focus, thus contributing to
developing competencies for long-term developments. Furthermore, they are pursuing business models
through technology adoption from open innovation and external/internal collaborations.
- Modified Drug Team :
-
primarily does research into dosage forms of new drugs and modifying new drugs and develop generic
products and OTC products. It also conducts research on how to improve the efficacy of existing drugs, and
development of drug control and release for patient convenience and composite drugs. With accumulated
technologies for drug development, it focuses on research into dosage forms that allow drugs to exhibit
outstanding effects of new drugs in human body. Plus, considering our buyers’ financial burden, we are
developing drugs whose safety and effectiveness is equivalent to that oforiginal products and various new
OTC products.
- Analytic Research Team :
-
uses excellent talents, experimental facilities and equipment, and accumulated know-hows and supports
efficient analytic research on drug development, develops new analytic methods for new materials and
dosage forms, and suggests criteria and analytic assessment methods for dosage forms. Furthermore,
it prepares news articles for new and existing products and undertakes safety tests and medical equivalency
tests for the maintenance and renew of national permit for our medical products.
- Natural Drug Team :
-
develops more standardized medicines through various manufacturing research including extracting and
refining natural components such as crude medicine, plant resources, and microorganism and accelerates
the development of high quality natural medicine featuring safety and outstanding efficacy. Through the
efforts, we aim at entering global natural medicine markets as well as domestic ones.
- Efficacy Assessment Team :
-
Efficacy Assessment Team is in charge of screening new elements in the early stage of the development
for synthetic and natural drugs and undertaking in vitro and in vivo drug assessment and safety tests.
Furthermore, it carries out non clinical research on effective elements and, with its distinguished
development of new drug, aims at contributing to the improvement of human health and welfare
through the development of safe and efficacious drugs
- Clinical Research Team :
-
undertakes clinical tests to prove safety and efficacy of medicine and medical equipment in accordance with
international standard GCP, domestic standard KGCP, and other relevant acts to clinical tests. Also, it is
leading tests considering the rights and benefits of test subjects thoroughly.